Through a tightly organized cascade of patterning, specification and differentiation events, the human brain develops into a highly complex system capable of unique cognitive abilities beyond those of other mammals. Although gene expression across the developing brain has been described at single-cell resolution, similar atlases of chromatin accessibility have been primarily focused on the regulatory landscape of the developing human forebrain in the second-trimester of pregnancy. A collaboration between Marijn Schipper and Danielle Posthuma from CNCR-CTG and Camiel Mannens and Sten Linnarsson from the Karolinska Institute Sweden has opened a new window in creating the first map of chromatin accessibility and paired gene expression in the entire embryonic human brain in the first three months of development. The study is published May 1, 2024 in Nature.
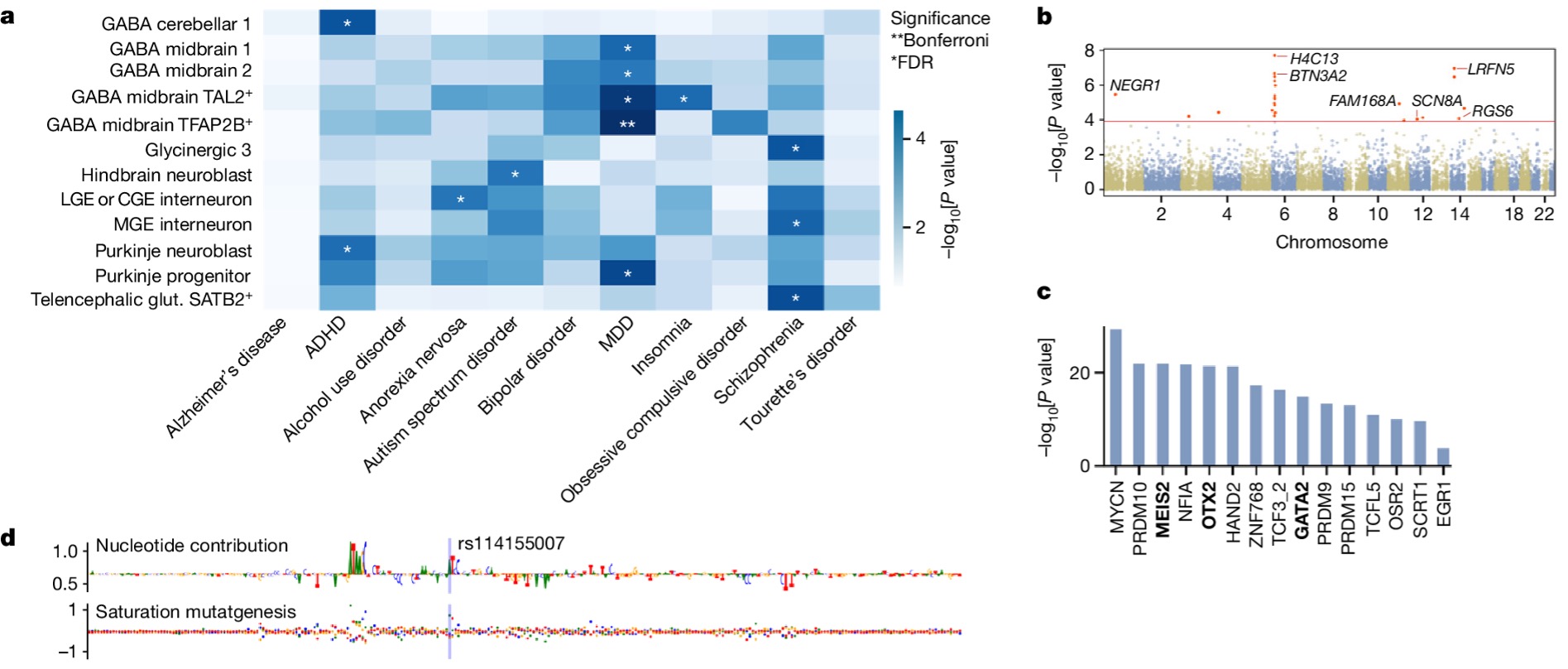
The high-resolution multi-omic atlas of chromatin accessibility and gene expression during the first-trimester of the developing human brain includes more than 100,000 cell-type- and region-specific developmental accessible chromatin regions, as well as inferred candidate cis-regulatory elements and their predicted regulatory effects. The resource allows for example linking transcription factors to putative enhancers, and enhancers to their target genes. In addition, this resource enables the interpretation of genetic associations with disease.
Marijn Schipper, PhD student at CNCR-CTG and part of the BRAINSCAPES consortium, investigated whether the parts of DNA that contain open chromatin in specific types of cells during early embryonic development overlap with genetic variants that have been identified previously in genome-wide association studies for psychiatric disorders. Marijn says: “we found that the patterns of open chromatin in the DNA in specific types of cells during development coincide with stretches of DNA that are also linked to ADHD, anorexia, autism spectrum disorders, depression, insomnia and schizophrenia. The strongest association was found for depression: genetic variants that have previously been linked to depression occur particularly frequently in stretches of DNA that are mainly active during early embryonic development in the GABAergic neurons in the midbrain. This link with the midbrain is novel, and offers new research directions with a specific focus on the midbrain for understanding the hereditary component of depression.”
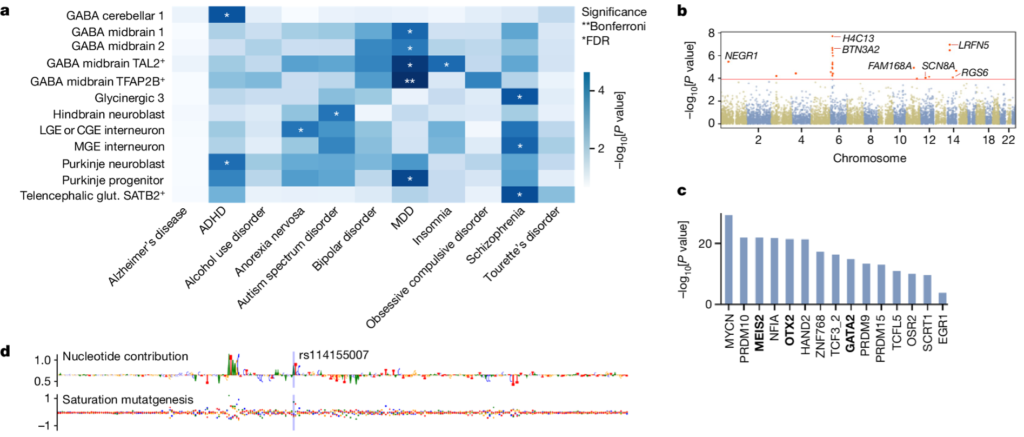
This work exemplifies how one of the main goals in BRAINSCAPES, linking GWAS results to actionable new insights into brain disorders, can be reached by using novel resources such as the developing brain open chromatin atlas. Additionally, the wealth of experience in linking GWAS to cell types present in BRAINSCAPES allows early career researchers such as Marijn Schipper to develop these skills and get involved in large scientific projects such as the chromatin-accessibility map project. This emphasizes the added value of our consortium and of course also the motivation and talent of our young researchers, which is something we are very proud of!
The paper can be found here: https://www.nature.com/articles/s41586-024-07234-1.