In their review recently published in Nature Reviews Neuroscience, Oxana Garritsen, Eljo van Battum, Laurens Grossouw and Jeroen Pasterkamp provide a comprehensive overview of the different molecular subsets and heterogeneity of dopaminergic neurons in the ventral midbrain of mice and humans. The authors first compare the currently defined subsets and then address molecular mechanisms that generate this diversity and that regulate subset positioning and target innervation in embryonic and early postnatal development. In addition, axonal wiring mechanisms and biological functions of dopaminergic neuron subtypes are extensively discussed. To further understand and treat diseases related to dopaminergic neurons, such as Parkinson disease, this review highlights the necessity of obtaining more extensive knowledge on midbrain dopamine neuron heterogeneity.
Dopamine neurons in the ventral midbrain (mDA neurons) are involved in the regulation of complex behaviours and locomotion and are affected in multiple brain disorders including Parkinson disease (PD). Historically, mDA neurons have been classified into three distinct regions primarily based on anatomical and cellular features: the retrorubral field, substantia nigra pars compacta (SNc) and ventral tegmental area (VTA). Previous research already revealed molecular differences between neurons in the VTA and SNc. Novel profiling studies of mDA neurons using single-cell sequencing techniques have even further identified the heterogeneity of mDA neurons. In parallel, mDA neurons are being classified using axonal tracing or functional approaches. Nevertheless, integration of data from these various approaches is challenging and far from complete. Pinpointing the differential susceptibility of mDA neuron subtypes to certain midbrain-associated disorders is, however, essential for generating and improving treatment strategies for these disorders. Therefore, establishing a comprehensive overview of mDA subsets, studying how these subsets arise and ultimately finding novel therapeutic targets are essential steps in this direction.
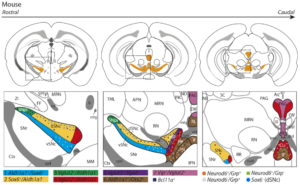
Heterogeneity in the mouse dopamine system
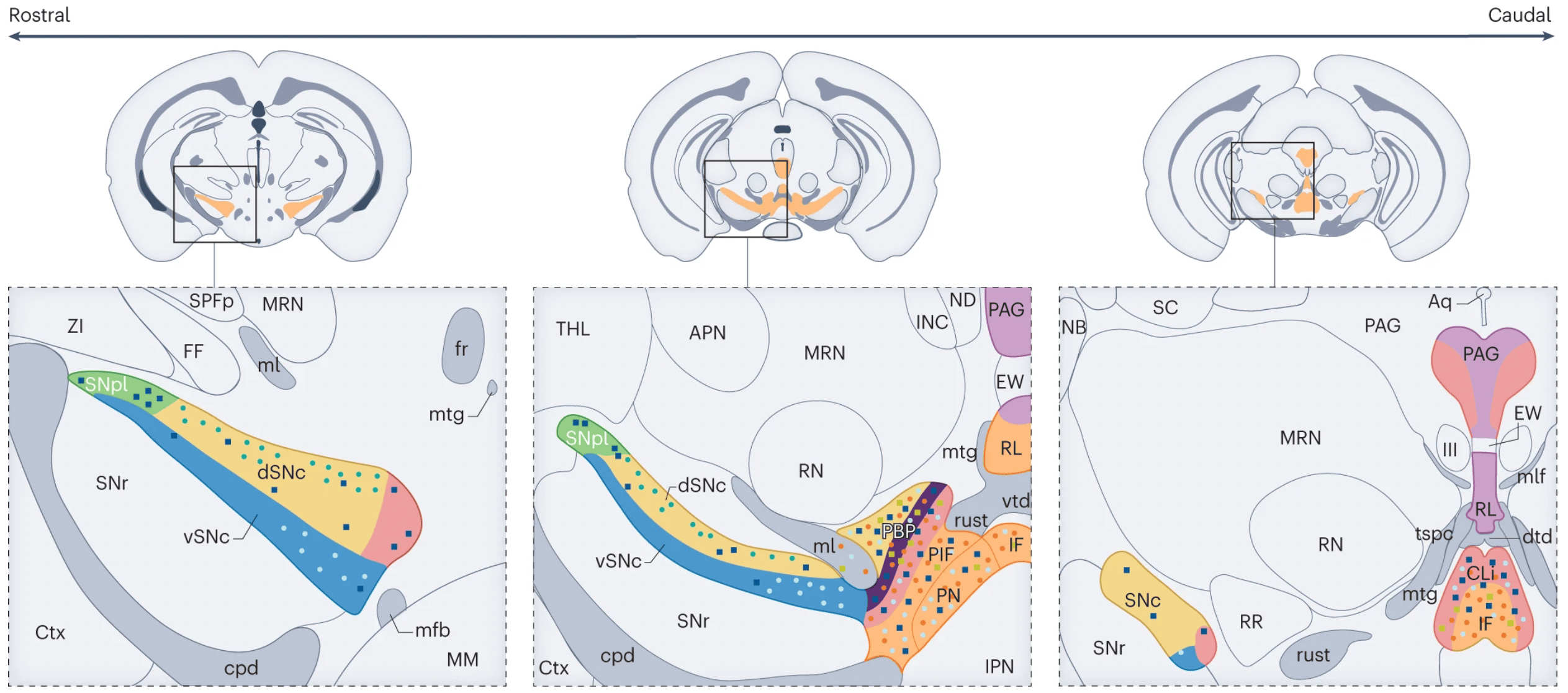
The review by Garritsen, van Battum, Grossouw et al., integrates transcriptomic studies to provide a detailed map of the currently identified mDA neuron subtypes. Building on previous literature, they show that the mDA system in mice is composed of at least seven subsets, of which three are located in the SNc and four in the VTA (see the figure on the left). In addition, several molecular subsets that have been identified in recent years reveal an additional layer of complexity on top of this classification. This indicates that the differences between mDA neurons in similar regions are yet to be fully understood. Comparing this with data from other species, including human transcriptomic data, suggested that similar subsets are likely also present in these species, although minor differences were observed, including species-specific subsets.
Establishing heterogeneity and target innervation during mouse development
Recent work has also begun to dissect molecular mechanisms instructing the development of subtypes within the VTA and SNc. In the review, it becomes clear that early during development specifically timed expression of combinations of transcription factors and morphogens already determines whether progenitor cells will become VTA or SNc neurons. This is then followed by specific migration patterns of the two distinct subsets. Due to the lack of specific (early) markers, however, studies regarding migration and specification of specific molecular mDA subtypes are still limited. Interestingly subtypes in the SNc are relatively segregated and anatomically confined to a subregion in the SNc while subtypes in the VTA are more intermingled and found throughout the entire VTA. Contrarily, both SNc and VTA subtypes project to very distinct regions and elicit specific functions, suggesting that specific molecular programs must be in place to establish these complex connectivity patterns. Although initial pathfinding might be regulated by a common mechanism, single-cell data indeed points towards differential expression of axon guidance molecules that could partially be responsible for the distinct patterns of target innervation.
Future implications for studies on midbrain dopaminergic neuron disorders
It is evident that subtypes of mDA neurons are differentially affected in disease. For example, specific projections to the rostral and dorsal caudate putamen show increased dopamine transmission in patients with schizophrenia and a specific subset of SNc neurons degenerate in PD. Further understanding of the molecular determinants during mDA subtype development and the mechanisms underlying their selective degeneration or resilience in PD is key in designing novel therapeutic strategies. Importantly, improved in vitro human disease models for specific dopaminergic subsets could refine cell replacement strategies, allowing transplantation of relevant subtypes only resulting in better treatment outcome.
The article can be read on: Development, wiring and function of dopamine neuron subtypes | Nature Reviews Neuroscience